自主神经系统由交感和副交感神经组成,其与疼痛存在密切关系。例如,当机体感受到疼痛刺激时会发生交感和副交感神经张力的改变,如心率、血压升高;同时,交感神经兴奋、副交感神经被抑制也在疼痛的产生中扮演重要角色[1]。
压力感受性反射是自主神经系统调节的重要机制,其在心肌梗死、心力衰竭等心血管疾病的预后预测中发挥重要作用[2-3]。近年来,越来越多的研究表明,压力反射敏感性(BRS)降低与慢性疼痛程度增加存在相关性[4-5]。这一关联为疼痛治疗提供了新策略:
1
BRS可作为疼痛管理的重要指标,筛查高危患者、评价治疗效果;
2
通过迷走神经刺激(VNS),改变压力感受性反射功能,起到减少炎症反应的作用,从而改善类风湿关节炎(RA)、系统性红斑狼疮(SLE)等风湿免疫病患者及术后患者的急慢性疼痛[6-9]。
本文就压力感受性反射在疼痛致病机制中的作用及其在疼痛治疗中的应用价值进行综述,以期为探索调节压力感受性反射功能的治疗手段提供新思路。
1 压力感受性反射
1.1 压力感受器与疼痛调控机制
人体内的压力感受器分布于颈动脉窦和主动脉弓,能够感知动脉血压改变,将信号传入中枢神经系统(CNS),通过神经反射途径调节心排出量、容量分布和动脉血压,以保持心血管系统的稳态平衡[10]。调节交感神经和副交感神经活性,影响心血管活动这一负反馈调节机制被称为压力感受性反射,是维持动脉血压水平相对稳定的重要机制之一。
压力感受器的末梢神经通过感受血管壁牵张程度,间接感知逐搏(beat-to-beat)动脉血压[11]。当动脉血压上升时,传入神经将信号传导至延髓,导致CNS中交感神经核团以及副交感神经核团反射性变化,交感神经活性减弱,副交感神经活性增强,心率减慢、心肌收缩力降低、心输出量减少,从而使动脉血压降低。当动脉血压下降时,神经核团将发生相反的变化,从而使动脉血压升高。
压力感受器由舌咽神经的颈动脉窦支支配,当感受到动脉血压改变时,神经元将神经冲动集中投射至延髓的孤束核。孤束核发出的上行纤维将神经冲动传导至延髓疑核,疑核心血管神经元发出投射至心脏的副交感节前神经,与胆碱能神经元形成突触,产生降低心率的效应[11]。
孤束核也可发出兴奋性谷氨酸能神经元,将神经冲动上传至延髓腹外侧尾端区(CVLM),进一步通过抑制性γ-氨基丁酸能神经元投射至延髓头端腹外侧区(RVLM),调节交感神经张力[11-12]。压力感受器将信号传导至孤束核后,这些通路通过平衡交感神经与副交感神经的张力,维持动脉血压相对稳定(图1)。
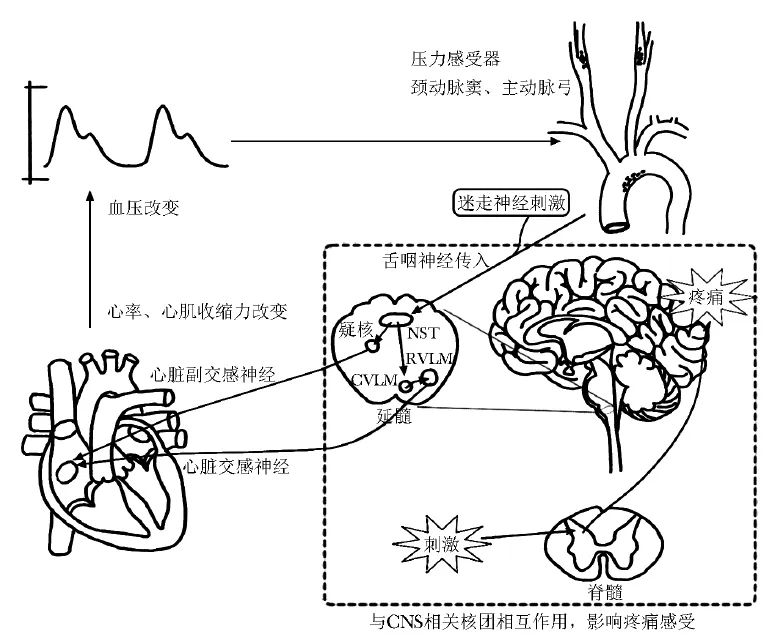
图1 压力感受性反射通路及影响疼痛的机制
上述与压力感受器相关的通路与疼痛调控有着密切联系。研究显示,当受试者接受到内脏传入神经刺激时,孤束核区域的活动程度显著增加[13]。孤束核、CVLM、杏仁核、丘脑/下丘脑、岛叶皮层、扣带回等结构之间形成的网络是自主神经与疼痛之间相互作用的关键结构[14-16]。Gu等[17]在小鼠实验中发现,激活CVLM的酪氨酸羟化酶阳性神经元可通过延髓腹外侧区-蓝斑核-脊髓环路,产生镇痛效应。
延髓头端腹内侧区也是CNS中调节伤害性感受信号的关键部位,该结构中的神经元能够直接投射至延髓及脊髓的心血管调控核团,进而降低副交感神经张力,从而使血压升高[18]。延髓头端腹内侧区与压力感受性反射通路中的孤束核、CVLM、RVLM等重要结构之间的相互联系及其在疼痛调节中的影响仍有待进一步明确。
1.2 压力反射敏感性测量
BRS是评估压力感受性反射功能的指标,能够定量反映人体压力感受性反射功能,其定义为血压变化引起心率反射性改变的敏感性,即心电图的RR间期[窦性心搏的RR间期,又被称为NN间期(normal-to-normal RR intervals)]与动脉收缩压变化的关系,单位为ms/mm Hg,即收缩压每变化1 mm Hg(1 mm Hg=0.133 kPa)时RR间期发生的变化。
最早的BRS测量方法为静脉注射少量肾上腺素α受体激动剂去氧肾上腺素的同时进行心电图和逐搏血压监测[19],计算线性RR间期变化与收缩压变化回归直线的斜率。由于该方法需要预先建立静脉和有创动脉监测通路,现多被更为简便易行的方法替代,包括Valsalva动作、颈部气囊加压[20-21]以及测量自发BRS。
压力感受性反射在日常活动中血压发生微小变化时也能通过调节心率使血压保持相对恒定,因此通过对逐搏血压和RR间期进行计算可得到自发BRS,自发BRS通常采用序列方法(sequence method)进行计算[22-23]。
传统的逐搏动脉压监测多采用桡动脉置管进行,近年连续无创血压(CNAP)监测技术被运用到BRS测量中,具有操作简单、无创的优势,且在预测有创BRS降低中具有良好的灵敏度和特异度[24-25]。作为压力感受性反射功能定性评价指标,BRS在疼痛严重程度预测、疼痛治疗效果评价中具有较大应用潜力,同时也可作为疼痛治疗中的靶点,为多模式镇痛提供新思路[26]。
2 压力感受性反射对痛觉的影响
疼痛已被证实与压力感受性反射功能密切相关。慢性高血压患者的疼痛耐受性升高[27-28],即使在血压正常的受试者中,疼痛敏感性同样与静息收缩压呈负相关[29-30]。在模型动物实验中,肾血管性高血压及遗传性高血压模型大鼠中均观察到脊髓痛觉传输信号的减弱[31-32]。
上述现象提示血压调节的CNS通路与疼痛调节的CNS通路有交叉关联。机体对短期内血压变化的调节与疼痛存在相关性,提示压力感受性反射在痛觉调控中产生了影响。BRS与疼痛敏感性的联系更加直接地支持了这一结论。在血压正常的受试者中进行的BRS测量及冷加压疼痛测试表明,BRS这一效应在静息血压较低者中被减弱[33]。这一机制可能是通过中枢敏化(即传入伤害性感受信号相关的功能增强)的减弱和下行抑制系统的激活实现[34]。因此,在急慢性疼痛患者中对压力感受性反射功能进行干预,可能有助于疼痛调控通路的激活,继而改善临床症状。
2.1 骨骼肌肉系统疾病的慢性疼痛
在纤维肌痛、颞下颌关节紊乱病(TMD)、慢性腰背痛等骨骼肌肉系统疾病中,压力感受性反射在慢性疼痛的发病机制中发挥重要作用[5,35-36]。在纤维肌痛患者中,临床慢性疼痛程度和疼痛敏感性测试均提示BRS与疼痛敏感性呈负相关[37-38]。压力感受性反射调节障碍可能是导致此类慢性疼痛疾病的共同病因[39]。
系统性炎症是自身免疫性疾病引起慢性疼痛的原因,作为免疫感受器,压力感受器在炎症反应的调节中起重要作用。炎症反应受神经系统调控,激活压力感受性反射通路能够降低炎症反应[40]。RA高风险者(RA相关血清抗体阳性)静息心率显著高于健康人群,且RA高风险者中静息心率更高者在随访期间发展为RA的风险更高,副交感胆碱能抗炎通路被抑制可能是原因之一[41]。交感神经张力降低、BRS较高的RA患者疼痛视觉模拟评分(VAS)更低,且与促炎细胞因子肿瘤坏死因子α(TNF-α)、IL-6水平降低也存在相关性[42-44]。
2.2 手术相关疼痛
围术期患者的自主神经功能可能存在复杂的变化,交感神经和副交感神经的张力受到手术应激、麻醉药物等多种因素影响[45-46]。与在健康受试者中观察到的现象类似,慢性高血压患者术后也存在疼痛耐受性增加的现象,经过抗高血压药物治疗后该效应减弱[47-49]。
有关术前BRS与术后疼痛相关性的研究结论尚未达成一致,针对开放腕管手术患者的研究认为,术前BRS与术后疼痛存在负相关[50],但针对心胸外科手术的研究认为二者存在正相关,此两种不一致的结论可能是由于不同手术类型对压力感受性反射通路产生的影响不同导致[51]。BRS降低可能与围术期不良事件、术后急慢性疼痛等转归具有密切关系,目前有关这一领域的研究较为有限,有待进一步深入探索。
3 压力感受性反射机制在疼痛治疗中的应用
3.1 迷走神经刺激
VNS是有效且安全的激活自主神经的方法,根据刺激部位不同分为耳分支或颈部分支刺激。VNS最早于1985年由Zabara[52]提出,其认为可通过刺激迷走神经,抑制神经元异常的超同步电活动,从而改善癫痫发作。
患者接受VNS时,迷走神经将信号传入中枢(图1),激活疼痛调节通路,因此VNS越来越多地被应用于急慢性疼痛的非药物治疗中。在纤维肌痛及SLE、RA等风湿免疫病患者中,VNS治疗能够明显改善疼痛症状和身体机能[53-54],降低患者血TNF-α、IL-6、IL-1β等炎症因子水平,并下调与痛觉传递有关的神经肽P物质的表达[7-8,55]。上述研究表明,VNS通过激活副交感胆碱能抗炎通路,从而改善患者的慢性疼痛,具有良好的应用前景。
VNS也被尝试用于围术期疼痛管理。2023年,Patel等[9]将经皮耳廓VNS应用于非心脏手术患者,分别于术前和术后24 h进行50 min的双侧VNS或假VNS,可显著缓解VNS组患者的术后疼痛,提高心率变异性(HRV),证实VNS可通过调节自主神经功能从而减轻围术期疼痛。
由此可见,VNS在已接受阿片类药物镇痛的患者中仍然能通过调控自主神经功能减少患者疼痛,成为围术期多模式镇痛的新选择,目前围术期VNS镇痛研究数量较为有限,仍需进一步探究刺激的最佳有效参数以及对术后慢性疼痛的影响。
3.2 麻醉药物
全麻中常用的镇痛药物对患者的围术期BRS存在明确影响。动物实验表明,大剂量强效阿片类镇痛药芬太尼可降低BRS[44],超短效阿片类镇痛药瑞芬太尼可导致交感神经活动增强[56]。
与阿片类药物的效应相反,输注右美托咪定能够保留全麻状态下犬的压力感受性反射功能,这种功能可能与全麻药物联合使用时才会凸显效应,单独使用右美托咪定在动物及临床研究中均未观察到BRS显著提高[57-58]。
右美托咪定是一种α2-肾上腺素受体激动剂,具有镇静镇痛作用。α2-肾上腺素受体在压力感受器相关疼痛调控通路中起到了关键作用,健康受试者中自发BRS介导的疼痛耐受性增加部分依靠肾上腺素α2受体相关机制介导[59]。
此外,去甲肾上腺素能神经元在上述延髓腹外侧区-蓝斑核-脊髓环路调控疼痛的机制中起到关键作用,而使用肾上腺素α2受体拮抗剂后镇痛效应消失[17]。
麻醉药物对BRS的改变可能对术后疼痛产生影响。Qiu等[60]研究显示,全麻术中泵注右美托咪定能够缓解大剂量瑞芬太尼导致的术后痛觉过敏,减少术后阿片类药物用量。这一现象可能与右美托咪定能够提高全麻期间BRS存在潜在关联,确切结论尚待进一步研究加以明确。
3.3 针灸
随着中医学的发展,针灸对自主神经系统的调节作用已被广泛认识。针刺特定穴位能够对杏仁核、室旁核、CVLM等与自主神经系统有重要关联的结构产生影响,调节自主神经功能,从而产生镇痛效果[61-62]。
例如,接受针刺内关穴的健康受试者心率降低、HRV上升,交感神经张力降低,副交感神经张力升高,且功能磁共振表明,CVLM、蓝斑核的激活是心血管自主神经变化的根源,也是调节疼痛传导通路的机制[63-64]。目前有关针灸对压力感受性反射通路直接影响的研究仍较少,需进一步探究针灸对此类核团的影响将通过何种方式调节自主神经功能。
4 小结与展望
压力感受性反射是自主神经系统调节心血管活动的重要机制,已被证实与疼痛调控具有密切关系。压力感受器传入信号对孤束核、延髓腹外侧区等结构中的核团产生影响,进而产生镇痛效应。
压力感受性反射功能可由BRS进行定量评估,大量研究证实,在健康受试者、慢性疼痛患者及手术患者中,BRS降低与疼痛敏感性升高存在相关性,因此BRS在临床疼痛风险筛查及疗效评估方面具有较大优势和潜力,有望成为辅助临床决策的重要参数。
在疼痛治疗方面,VNS通过调节自主神经功能,起到镇痛、抗炎作用,治疗效果已在各种急慢性疼痛患者中得到证实,在疼痛的非药物治疗中有望进一步推广。右美托咪定、针灸治疗也被认为可能与自主神经调节有关,能够起到镇痛作用。
目前研究中,关于压力感受性反射与起到调节伤害性感受信号作用的延髓头端腹内侧区存在何种直接联系的证据,以及BRS介导的镇痛效应的数据较少。相信随着研究的进一步深入,以及更多循证医学证据的不断涌现,急慢性疼痛患者的临床治疗将变得更加高效与精准。
参考文献
[1]Bantel C, Trapp S. The role of the autonomic nervous system in acute surgical pain processing-what do we know?[J]. Anaesthesia, 2011, 66(7): 541-544.
[2]Giannoni A, Gentile F, Buoncristiani F, et al. Chemoreflex and baroreflex sensitivity hold a strong prognostic value in chronic heart failure[J]. JACC Heart Fail, 2022, 10(9): 662-676.
[3]Kaufmann D K, Raczak G, Szwoch M, et al. Baroreflex sensitivity but not microvolt T-wave alternans can predict major adverse cardiac events in ischemic heart failure[J]. Cardiol J, 2022, 29(6): 1004-1012.
[4]Davydov D M, Naliboff B, Shahabi L, et al. Asymmetries in reciprocal baroreflex mechanisms and chronic pain severity: focusing on irritable bowel syndrome[J]. Neurogastroen-terol Motil, 2018, 30(2): e13186.
[5]Reyes Del Paso G A, Contreras-Merino A M, De La Coba P, et al. The cardiac, vasomotor, and myocardial branches of the baroreflex in fibromyalgia: associations with pain, affective impairments, sleep problems, and fatigue[J]. Psychophysiology, 2021, 58(5): e13800.
[6]Yuan H, Silberstein S D. Vagus nerve and vagus nerve stimulation, a comprehensive review: part III[J]. Headache, 2016, 56(3): 479-490.
[7]Koopman F A, Chavan S S, Miljko S, et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis[J]. Proc Natl Acad Sci U S A, 2016, 113(29): 8284-8289.
[8]Aranow C, Atish-Fregoso Y, Lesser M, et al. Transcutane-ous auricular vagus nerve stimulation reduces pain and fatigue in patients with systemic lupus erythematosus: a randomised, double-blind, sham-controlled pilot trial[J]. Ann Rheum Dis, 2021, 80(2): 203-208.
[9]Patel A B U, Bibawy P P W M, Althonayan J I M, et al. Effect of transauricular nerve stimulation on perioperative pain: a single-blind, analyser-masked, randomised control-led trial[J]. Br J Anaesth, 2023, 130(4): 468-476.
[10]Rabinovitch A, Friedman M, Braunstein D, et al. The baroreflex mechanism revisited[J]. Bull Math Biol, 2015, 77(8): 1521-1538.
[11]Saper C B. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation[J]. Annu Rev Neurosci, 2002, 25: 433-469.
[12]Weston M, Wang H, Stornetta R L, et al. Fos expression by glutamatergic neurons of the solitary tract nucleus after phenylephrine-induced hypertension in rats[J]. J Comp Neurol, 2003, 460(4): 525-541.
[13]Beckers A B, Van Oudenhove L, Weerts Z Z R M, et al. Evidence for engagement of the nucleus of the solitary tract in processing intestinal chemonociceptive input irrespective of conscious pain response in healthy humans[J]. Pain, 2022, 163(8): 1520-1529.
[14]Benarroch E E. Pain-autonomic interactions[J]. Neurol Sci, 2006, 27(Suppl 2): S130-S133.
[15]He S L, Huang X L, Zheng J, et al. An NTS-CeA projection modulates depression-like behaviors in a mouse model of chronic pain[J]. Neurobiol Dis, 2022, 174: 105893.
[16]Marques-Lopes J, Pinto M, Pinho D, et al. Microinjec-tion of angiotensin II in the caudal ventrolateral medulla induces hyperalgesia[J]. Neuroscience, 2009, 158(4): 1301-1310.
[17]Gu X L, Zhang Y Z, O'Malley J J, et al. Neurons in the caudal ventrolateral medulla mediate descending pain control[J]. Nat Neurosci, 2023, 26(4): 594-605.
[18]Babic T, Ciriello J. Medullary and spinal cord projections from cardiovascular responsive sites in the rostral ventro-medial medulla[J]. J Comp Neurol, 2004, 469(3): 391-412.
[19]Eckberg D L, Drabinsky M, Braunwald E. Defective cardiac parasympathetic control in patients with heart disease[J]. N Engl J Med, 1971, 285(16): 877-883.
[20]Raczak G, La Rovere M T, Pinna G D, et al. Assessment of baroreflex sensitivity in patients with preserved and impaired left ventricular function by means of the Valsalva manoeuvre and the phenylephrine test[J]. Clin Sci (Lond), 2001, 100(1): 33-41.
[21]Bernardi L, Bianchini B, Spadacini G, et al. Demonstrable cardiac reinnervation after human heart transplantation by carotid baroreflex modulation of RR interval[J]. Circulation, 1995, 92(10): 2895-2903.
[22]La Rovere M T, Specchia G, Mortara A, et al. Barore-flex sensitivity, clinical correlates, and cardiovascular mortality among patients with a first myocardial infarction. A prospective study[J]. Circulation, 1988, 78(4): 816-824.
[23]Petry D, Mirian De Godoy Marques C, Brum Marques J L. Baroreflex sensitivity with different lags and random forests for staging cardiovascular autonomic neuropathy in subjects with diabetes[J]. Comput Biol Med, 2020, 127: 104098.
[24]Madden K M, Feldman B, Meneilly G S. Baroreflex function and postprandial hypotension in older adults[J]. Clin Auton Res, 2021, 31(2): 273-280.
[25]Hartikainen J E, Tahvanainen K U, Mntysaari M J, et al. Simultaneous invasive and noninvasive evaluations of baroreflex sensitivity with bolus phenylephrine technique[J]. Am Heart J, 1995, 130(2): 296-301.
[26]Thieme K, Jung K, Mathys M G, et al. Cardiac-gated neuromodulation increased baroreflex sensitivity and reduced pain sensitivity in female fibromyalgia patients[J]. J Clin Med, 2022, 11(20): 6220.
[27]Ghione S, Rosa C, Mezzasalma L, et al. Arterial hyperten-sion is associated with hypalgesia in humans[J]. Hypertension, 1988, 12(5): 491-497.
[28]Nascimento Rebelatto M, Alburquerque-Sendín F, Guimarães J F, et al. Pressure pain threshold is higher in hypertensive compared with normotensive older adults: a case-control study[J]. Geriatr Gerontol Int, 2017, 17(6): 967-972.
[29]Bruehl S, Burns J W, Chung O Y, et al. Hypoalgesia associated with elevated resting blood pressure: evidence for endogenous opioid involvement[J]. J Behav Med, 2010, 33(2): 168-176.
[30]Bruehl S, Carlson C R, McCubbin J A. The relationship between pain sensitivity and blood pressure in normotensives[J]. Pain, 1992, 48(3): 463-467.
[31]Randich A, Robertson J D. Spinal nociceptive transmission in the spontaneously hypertensive and Wistar-Kyoto normotensive rat[J]. Pain, 1994, 58(2): 169-183.
[32]Zamir N, Segal M. Hypertension-induced analgesia: changes in pain sensitivity in experimental hypertensive rats[J]. Brain Res, 1979, 160(1): 170-173.
[33]Duschek S, Mück I, Reyes Del Paso G A. Relationship between baroreceptor cardiac reflex sensitivity and pain experience in normotensive individuals[J]. Int J Psychophysiol, 2007, 65(3): 193-200.
[34]Chung O Y, Bruehl S, Diedrich L, et al. The impact of blood pressure and baroreflex sensitivity on wind-up[J]. Anesth Analg, 2008, 107(3): 1018-1025.
[35]Bruehl S, Chung O Y, Ward P, et al. The relationship between resting blood pressure and acute pain sensitivity in healthy normotensives and chronic back pain sufferers: the effects of opioid blockade[J]. Pain, 2002, 100(1/2): 191-201.
[36]Maixner W, Greenspan J D, Dubner R, et al. Potential autonomic risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study[J]. J Pain, 2011, 12(11 Suppl): T75-T91.
[37]Reyes Del Paso G A, Garrido S, Pulgar Á, et al. Autonomic cardiovascular control and responses to experimental pain stimulation in fibromyalgia syndrome[J]. J Psychosom Res, 2011, 70(2): 125-134.
[38]Zamunér A R, Barbic F, Dipaola F, et al. Relationship between sympathetic activity and pain intensity in fibromyalgia[J]. Clin Exp Rheumatol, 2015, 33(1 Suppl 88): S53-S57.
[39]Light K C, Bragdon E E, Grewen K M, et al. Adrenergic dysregulation and pain with and without acute beta-blockade in women with fibromyalgia and temporomandibular disorder[J]. J Pain, 2009, 10(5): 542-552.
[40]Brognara F, Castania J A, Kanashiro A, et al. Physiological sympathetic activation reduces systemic inflammation: role of baroreflex and chemoreflex[J]. Front Immunol, 2021, 12: 637845.
[41]Koopman F A, Tang M W, Vermeij J, et al. Autonomic dysfunction precedes development of rheumatoid arthritis: a prospective cohort study[J]. EBioMedicine, 2016, 6: 231-237.
[42]Bernstein I M, Damron D, Schonberg A L, et al. The relationship of plasma volume, sympathetic tone, and proinflammatory cytokines in young healthy nonpregnant women[J]. Reprod Sci, 2009, 16(10): 980-985.
[43]Pöyhönen-Alho M K, Manhem K, Katzman P, et al. Central sympatholytic therapy has anti-inflammatory properties in hypertensive postmenopausal women[J]. J Hypertens, 2008, 26(12): 2445-2449.
[44]Adlan A M, Paton J F R, Lip G Y H, et al. Increased sympathetic nerve activity and reduced cardiac baroreflex sensitivity in rheumatoid arthritis[J]. J Physiol, 2017, 595(3): 967-981.
[45]Dorantes Mendez G, Aletti F, Toschi N, et al. Baroreflex sensitivity variations in response to propofol anesthesia: comparison between normotensive and hypertensive patients[J]. J Clin Monit Comput, 2013, 27(4): 417-426.
[46]臧晗, 余佳文, 车璐, 等. 自主神经功能对全身麻醉诱导后低血压的预测作用[J]. 医学研究杂志, 2023, 52(6): 190-193.
[47]France C R, Katz J. Postsurgical pain is attenuated in men with elevated presurgical systolic blood pressure[J]. Pain Res Manag, 1999, 4: 460391.
[48]Pan P H, Coghill R, Houle T T, et al. Multifactorial preoperative predictors for postcesarean p pain and analgesic requirement[J]. Anesthesiology, 2006, 104(3): 417-425.
[49]Chiang H L, Huang Y C, Lin H S, et al. Hypertension and postoperative pain: a prospective observational study[J]. Pain Res Manag, 2019, 2019: 8946195.
[50]Nielsen R, Nikolajsen L, Krøner K, et al. Pre-operative baroreflex sensitivity and efferent cardiac parasympathetic activity are correlated with post-operative pain[J]. Acta Anaesthesiol Scand, 2015, 59(4): 475-485.
[51]Suarez-Roca H, Mamoun N, Watkins L L, et al. Higher cardiovagal baroreflex sensitivity predicts increased pain outcomes after cardiothoracic surgery[J]. J Pain, 2024, 25(1): 187-201.
[52]Zabara J. Peripheral control of hypersynchronous discharge in epilepsy[J]. Electroencephalogr Clin Neurophysiol, 1985, 61(3): S162.
[53]Lange G, Janal M N, Maniker A, et al. Safety and efficacy of vagus nerve stimulation in fibromyalgia: a phase Ⅰ/Ⅱ proof of concept trial[J]. Pain Med, 2011, 12(9): 1406-1413.
[54]Kutlu N, Özden A V, Alptekin H K, et al. The impact of auricular vagus nerve stimulation on pain and life quality in patients with fibromyalgia syndrome[J]. Biomed Res Int, 2020, 2020: 8656218.
[55]Drewes A M, Brock C, Rasmussen S E, et al. Short-term transcutaneous non-invasive vagus nerve stimulation may reduce disease activity and pro-inflammatory cytokines in rheumatoid arthritis: results of a pilot study[J]. Scand J Rheumatol, 2021, 50(1): 20-27.
[56]Shirasaka T, Yano T, Kunitake T, et al. High-dose remifentanil increases blood pressure and heart rate mediated by sympatho-activation in conscious rats[J]. J Anesth, 2013, 27(3): 325-332.
[57]Hogue C W, Jr, Talke P, Stein P K, et al. Autonomic nervous system responses during sedative infusions of dexmedetomidine[J]. Anesthesiology, 2002, 97(3): 592-598.
[58]Devcic A, Schmeling W T, Kampine J P, et al. Oral dexmedetomidine preserves baroreceptor function and decreases anesthetic requirements of halothane-anesthetized dogs[J]. Anesthesiology, 1994, 81(2): 419-430.
[59]Chung O Y, Bruehl S, Diedrich L, et al. Baroreflex sensitivity associated hypoalgesia in healthy states is altered by chronic pain[J]. Pain, 2008, 138(1): 87-97.
[60]Qiu H D, Sun Z, Shadhiya F, et al. The influence of dexmedetomidine on remifentanil-induced hyperalgesia and the sex differences[J]. Exp Ther Med, 2018, 16(4): 3596-3602.
[61]Li Y W, Li W, Wang S T, et al. The autonomic nervous system: a potential link to the efficacy of acupuncture[J]. Front Neurosci, 2022, 16: 1038945.
[62]Pang Y, Liao H, Duan G X, et al. Regulated aberrant amygdala functional connectivity in premenstrual syndrome via electro-acupuncture stimulation at Sanyinjiao acupoint (SP6)[J]. Gynecol Endocrinol, 2021, 37(4): 315-319.
[63]Dhond R P, Yeh C, Park K, et al. Acupuncture modulates resting state connectivity in default and sensorimotor brain networks[J]. Pain, 2008, 136(3): 407-418.
[64]Beissner F, Deichmann R, Henke C, et al. Acupuncture--deep pain with an autonomic dimension?[J]. Neuroimage, 2012, 60(1): 653-660.