[摘要] 背景与目的:局部进展期直肠癌(locally advanced rectal cancer,LARC)的标准治疗策略是新辅助放化疗(neoadjuvant chemoradiotherapy,nCRT)后进行手术治疗,nCRT可以使肿块缩小,实现肿瘤降期,增加R0切除率。但直肠癌个体差异较大,有部分患者对nCRT反应较差,并不能从nCRT中获益。因此,采取有效的筛选措施,以识别nCRT效果不佳的患者很有必要。本研究旨在探讨临床基线指标对LARC nCRT后肿瘤退缩的预测价值并构建肿瘤退缩预测模型。方法:收集2016年1月—2020年12月在空军军医大学第一附属医院接受nCRT治疗且行全直肠系膜切除术的LARC患者,收集入组患者nCRT前的临床基线指标,包括实验室检查、肿瘤标志物和磁共振成像(magnetic resonance imaging,MRI)资料。根据nCRT前后的MRI报告的肿瘤大小,通过实体瘤疗效评价标准(Response Evaluation Criteria in Solid Tumors,RECIST)来评价LARC患者nCRT后肿瘤退缩程度。使用受试者工作特征(receiver operating characteristic,ROC)曲线对临床基线指标标准化处理后,进行多因素分析,筛选影响肿瘤退缩的因素,通过logistic回归构建肿瘤退缩预测模型,采用决策曲线分析(decision curve analysis,DCA)和校准曲线对模型的预测性能进行评估,并通过十折交叉验证来检测模型的准确度。结果:本回顾性队列研究共入组158例患者,其中98例患者nCRT后肿瘤退缩良好,达到完全缓解(complete response,CR)或部分缓解(partial response,PR),客观缓解率为62%;60例患者退缩不良,为疾病稳定(stable disease,SD)或疾病进展(progressive disease,PD)。多因素分析表明,治疗前肿瘤直径(P<0.001)、nCRT后距离手术的时间(P=0.006)、D-二聚体(P=0.010)、预后营养指数(prognostic nutrition index,PNI)(P=0.035)、癌胚抗原(carcinoembryonic antigen,CEA)(P=0.004)、壁外血管侵犯(extramural vascular invasion,EMVI)(P=0.026)与nCRT后肿瘤退缩显著相关。LARC nCRT后肿瘤退缩预测模型的ROC曲线的曲线下面积(area under curve,AUC)为0.84 (95% CI:0.780~0.899),灵敏度为85.0%,特异度为72.4%。在校准曲线中,预测结果与实际结果吻合良好,具有良好的预测精准度。DCA表明,肿瘤退缩预测模型可以为诊断带来临床净收益。结论:治疗前肿瘤直径、nCRT后距离手术的时间、D-二聚体、PNI、CEA及EMVI是LARC nCRT后肿瘤退缩的独立风险因素,基于上述因素构建的肿瘤退缩预测模型对LARC患者nCRT后的肿瘤退缩具有较好的预测效能。
[关键词] 直肠癌;局部进展期;新辅助放化疗;肿瘤退缩预测
[Abstract] Background and purpose: The standard therapy for locally advanced rectal cancer (LARC) is neoadjuvant chemoradiotherapy (nCRT) followed by surgery. NCRT can make the tumor regress and downstage, and increase the R0 rep rate. However, individual differences in rectal cancer are large, and some patients respond poorly to nCRT and cannot benefit from nCRT. Therefore, it is necessary to establish effective screening measures to identify patients with poor response to nCRT. This study aimed to analyze the influencing factors of nCRT for LARC and construct the tumor regression prediction model. Methods: Data of 158 LARC patients who underwent total mesenteric rep after receiving nCRT at the First Hospital Affiliated to Air Force Medical University from January 2016 to December 2020 were collected. Baseline clinical indicators before nCRT were collected, including laboratory examination, tumor markers and magnetic resonance imaging (MRI). According to the tumor size reported by MRI before and after nCRT, Response Evaluation Criteria in Solid Tumors (RECIST) was used to evaluate the extent of tumor regression after nCRT. After receiver operating characteristic (ROC) curve was used to standardize the clinical baseline indicators, logistic regression analysis was carried out to screen the factors affecting the tumor regression. The tumor regression prediction model was constructed by logistic regression, and the performance of the model was evaluated based on decision curve analysis (DCA) and the calibration curve. The accuracy of the model was tested by 10-fold cross-validation. Results: This retrospective cohort study enrolled 158 patients, in which, 98 patients achieved complete response (CR) or partial response (PR). The objective response rate was 62%. Sixty patients had poor response to nCRT, either stable disease (SD) or progressive disease (PD). Multivariate logistic regression analysis showed that tumor diameter before treatment (P<0.001), time to surgery after nCRT (P=0.006), D-dimer (P=0.010), prognostic nutrition index (PNI) (P=0.035), carcinoembryonic antigen (CEA) (P=0.004) and extramural vascular invasion (EMVI) (P=0.026) were significantly related to tumor regression after nCRT. The area under ROC curve (AUC) of tumor regression after nCRT prediction model for LARC was 0.84 (95% CI: 0.780-0.899), sensitivity was 85.0%, and specificity was 72.4%. In the calibration curve, the predicted results were in good agreement with the actual results, and the prediction accuracy was good. The DCA showed that the tumor regression prediction model could bring clinical net benefit to diagnosis. Conclusion: Tumor diameter before treatment, time to surgery after nCRT, D-dimer, PNI, CEA and EMVI are independent risk factors for the tumor regression after nCRT in LARC patients. The tumor regression prediction model based on the above factors has good predictive efficacy for the tumor regression after nCRT in LARC patients.
[Key words] Rectal cancer; Local advanced; Neoadjuvant chemoradiotherapy; Tumor regression prediction
2020年全世界新增约73万例直肠癌新发病例及34万例死亡病例,分别占癌症新发病例和死亡病例的3.8%和3.4%[1]。直肠癌早期无明显症状,超过38%的患者确诊时已发展至局部进展期,5年生存率为32.8%~61.4%[2-3]。目前,局部进展期直肠癌(locally advanced rectal cancer,LARC)的标准治疗策略是新辅助放化疗(neoadjuvant chemoradiotherapy,nCRT)联合直肠系膜切除术(total mesorectal excision,TME)[4],nCRT可以显著提高局部控制率,使肿瘤降期以达到R0切除[5-6],但是LARC存在异质性,临床上不同患者对nCRT的反应差异显著,只有15%~27%的患者能在nCRT后实现病理学完全缓解(pathologic complete response,pCR)[7],约60%的患者在nCRT后实现肿瘤退缩可以手术切除,还有20%对治疗反应不敏感或疾病进展导致无法手术[8-9]。另外接近20%的患者出现治疗相关不良反应,如疲劳、放射性直肠炎和骨髓抑制等[10]。因此,准确地识别nCRT后肿瘤退缩不良的患者,有助于实现个体化精准治疗。本研究回顾性分析接受nCRT的LARC患者的临床基线指标,探讨影响LARC患者nCRT后肿瘤退缩的基线因素并构建肿瘤退缩预测模型,旨在为临床精准化治疗提供参考。
1 资料和方法
1.1 研究对象
回顾性分析2016年1月—2020年12月在空军军医大学第一附属医院接受nCRT且行全直肠系膜切除术的LARC患者。病例纳入标准:① 肿瘤距肛门距离≤15 cm;② 治疗前磁共振成像(magnetic resonance imaging,MRI)提示T3/T4期或N+期、无远处转移;③ 患者仅存在直肠癌,无其他恶性肿瘤;④ 术前均接受nCRT,无手术禁忌证;⑤ 病例资料完整。排除标准:① 依从性差或身体原因未接受完整nCRT的患者;② nCRT期间因急症(如肠梗阻)行急诊手术的患者;③ 患者治疗前存在慢性或急性感染等全身性炎症;④ 患者有过敏性疾病、血液系统疾病;⑤ 治疗前持续服用抗凝、抗血小板、升白细胞等影响血液系统指标的药物;⑥ 患者资料不全或在外院接受nCRT。本研究经过空军军医大学第一附属医院医学伦理委员会批准(批准文号:XJLL-KY-20222314),免除受试者知情同意。
1.2 nCRT方案
术前均进行长程放化疗:盆腔照射剂量为45~50 Gy,每周5次,共25次。放疗期间同步进行化疗,44例接受XELOX方案治疗(奥沙利铂130 mg/m2第1天+卡培他滨1 000 mg/m2第1~14天,每天2次,方案每3周1次),114例接受卡培他滨单药(825 mg/m2,每天2次)治疗。
1.3 nCRT后肿瘤退缩程度评定
通过对比患者nCRT前后的MRI资料,根据实体瘤疗效评价标准(Response Evaluation Criteria in Solid Tumors,RECIST)[11]对nCRT后肿瘤退缩程度进行评价:
① 完全缓解(complete response,CR):所有靶病灶消失,无新病灶出现,且肿瘤标志物正常,至少维持4周。② 部分缓解(partial response,PR):靶病灶最大径之和减少≥30%,至少维持4周。③ 疾病稳定(stable disease,SD):靶病灶最大径之和缩小未达PR,或增大但未达PD。④ 疾病进展(progressive disease,PD):靶病灶最大径之和至少增加≥20%,或出现新病灶。
1.4 统计学处理
数据采用R 4.1.2软件进行统计学分析。分类变量采用χ2检验或Fisher精确检验,连续变量采用独立样本t检验。计量数据符合正态分布用x±s表示;非正态分布的计量数据用M(P25,P75)表示;计数资料用n(%)表示。进行单因素logistic回归和多因素logistic回归分析筛选与肿瘤退缩相关的基线特征。采用R软件“pROC”包确定基线资料的最佳截断值,将连续性变量转化为二分类变量;采用R软件“rms”包进行列线图绘制。P<0.05为差异有统计学意义。
2 结 果
2.1 临床病理学特征
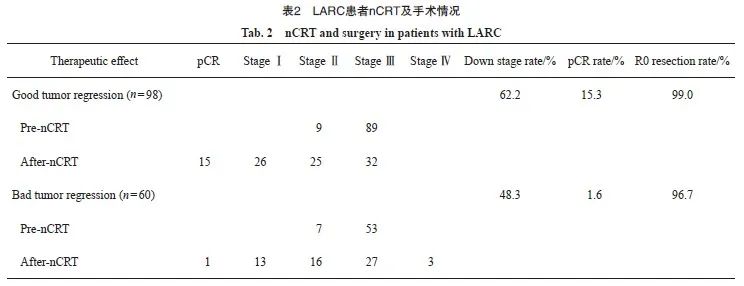
本研究共入组158例LARC患者,分为退缩良好组(CR+PR,n=98)和退缩不良组(SD+PD,n=60),两组的临床基线指标见表1。nCRT后降期的患者,退缩良好组61例(62.2%),退缩不良组29例(48.3%),两组的pCR率分别为15.3%和1.6%,R0切除率分别为99.0%和96.7%(表2)。单因素logistic回归分析显示,两组间在治疗前肿瘤直径、癌胚抗原(carcinoembryonic antigen,CEA)、预后营养指数(prognostic nutrition index,PNI)、白蛋白、D-二聚体、纤维蛋白原、纤维蛋白原/白蛋白比值(fibrinogen/albumin ratio,FAR)、壁外血管侵犯(extramural vascular invasion,EMVI)及nCRT后距离手术的时间方面差异有统计学意义(P<0.05)。多因素logistic回归分析显示,治疗前肿瘤直径(P<0.001)、nCRT后距离手术的时间(P=0.006)、D-二聚体(P=0.010)、PNI(P=0.035)、CEA(P=0.004)、EMVI(P=0.026)与nCRT后肿瘤退缩显著相关(表3)。

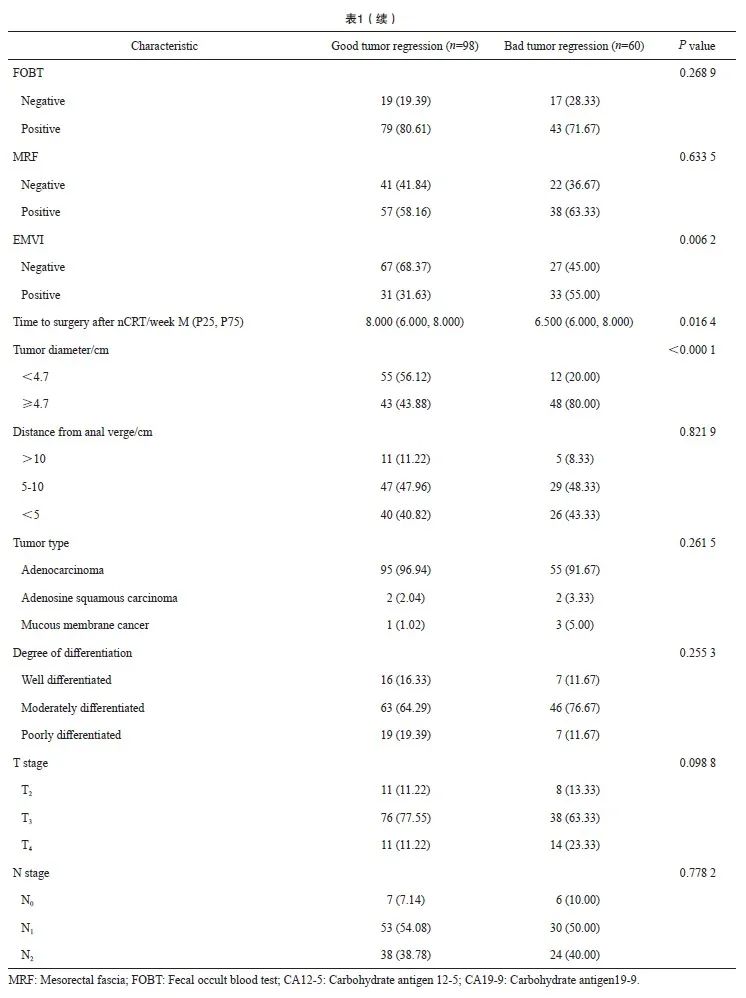
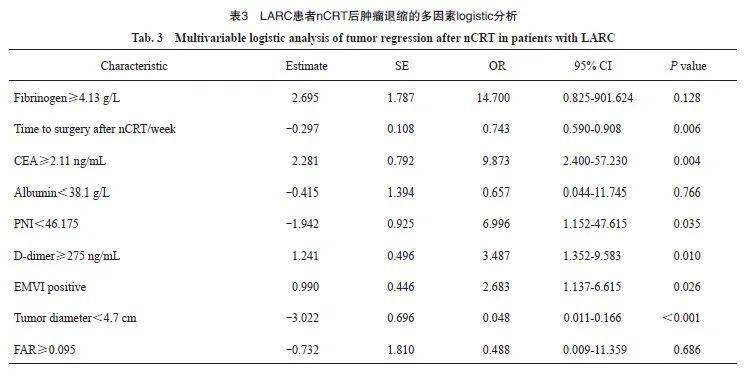
2.2 预测模型的效能
基于多因素logistic回归分析的结果,使用R软件构建LARC nCRT后肿瘤退缩预测模型。受试者工作特征(receiver operating characteristic,ROC)曲线的曲线下面积(area under curve,AUC)为0.84(95% CI:0.780~0.899),灵敏度为85.0%,特异度为72.4%(图1),提示肿瘤退缩预测模型的预测效能良好。

图1 肿瘤退缩预测模型的ROC曲线
Fig. 1 ROC curves for tumor regression prediction model
2.3 预测模型的验证
本研究采用了十折交叉验证的方法来验证预测模型的性能,其准确度为0.73,一致度为0.42,具有良好的预测能力。在校准曲线中,预测结果与实际结果吻合良好,表明预测模型预测nCRT后肿瘤退缩与实际差异无统计学意义(P>0.05,图2);决策曲线分析(decision curve analysis,DCA)表明,随着风险阈值升高,模型均可以获益,预测模型可以为诊断带来临床净收益(图3)。
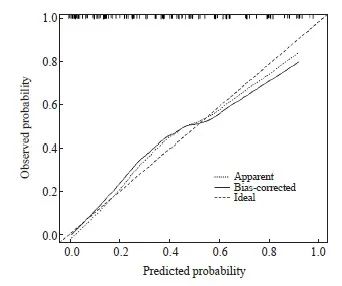
图2 肿瘤退缩预测模型的校准曲线
Fig. 2 Calibration curve of tumor regression prediction model
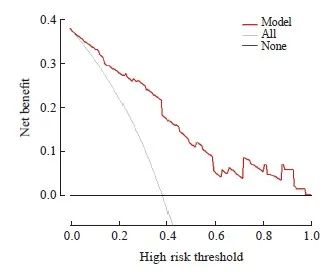
图3 肿瘤退缩预测模型的DCA
Fig. 3 DCA for tumor regression prediction model
2.4 亚组分析
本研究的术前nCRT方案主要为卡培他滨单药方案(n=114)和XELOX方案(n=44),肿瘤退缩分析见表4。利用两组患者分别对预测模型进行验证,卡培他滨单药方案组的AUC为0.825 (95% CI:0.751 2~0.899 6),XELOX方案组的AUC为0.921(95% CI:0.845 3~0.996 6),均得到可靠的AUC(图4)。在本研究的数据集中, nCRT方案并不会影响预测模型的效能。
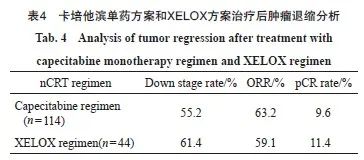
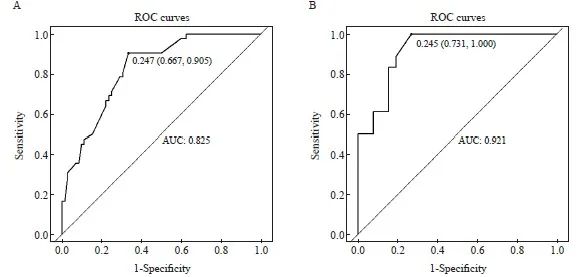
图4 卡培他滨单药方案组(A)和XELOX方案组(B)的ROC曲线
Fig. 4 ROC curves of capecitabine monotherapy regimen group (A) and XELOX regimen group (B)
2.5 列线图构建
通过列线图的构建,使预测模型可视化,综合体现模型中变量对nCRT后肿瘤退缩的预测价值(图5)。图中左侧变量有相对应的点,即该变量的得分,将所有变量得分相加得到总分,总分相对应的概率即nCRT后肿瘤退缩不良的预测概率。
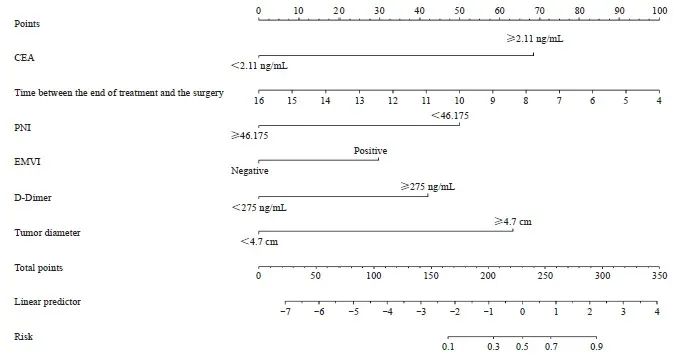
图5 肿瘤退缩预测模型的列线图
Fig. 5 Nomogram of the tumor regression prediction model
3 讨 论
本研究基于临床基线指标构建了一个LARC nCRT后肿瘤退缩预测模型。nCRT可以显著地提高LARC的局部控制率,但因为肿瘤的异质性,并不是所有患者都对nCRT反应良好[12]。对于肿瘤退缩不良的部分患者,继续进行nCRT意味着不必要的药物毒性和治疗延误。本研究提出的模型可以对LARC患者的治疗反应进行较为精准的预测,对作出临床决策和明确治疗方向有重要 意义。
本研究对nCRT后肿瘤退缩的临床病理学特征的分析结果与既往研究一致。肿瘤大小、CEA及EMVI与直肠癌的预后及nCRT效果显著相关[13-14]。直肠癌中约有80%为腺癌,直肠黏液腺癌也被视为术前放化疗反应不良的生物标志物[15],而本研究中超过95%的患者为直肠腺癌,所以本研究中的组织学分类并未显示出与肿瘤退缩的相关性;关于T分期及N分期与肿瘤退缩预测效能的研究报道不一,可能由于样本量较小,本研究中T分期及N分期并不能作为预测肿瘤退缩的因素[16-18]。另外,国外一项随机临床试验[19]结果显示,术前nCRT后距离手术的时间与肿瘤退缩有关,表现为在手术间隔为0 ~ 15周,随着间隔时间延长,达到pCR的机会明显增加,这与本研究结果一致;在一定的范围内,可以通过延长治疗后距离手术的时间,期待肿瘤的进一步消退甚至达到pCR。另外,本研究还确定了几个与nCRT后肿瘤退缩相关的营养炎症因素,包括PNI、D-二聚体。近年来,多项研究[20-21]证明,癌症与高凝状态有关。D-二聚体是纤维蛋白的降解产物,同时也是凝血激活的信号。D-二聚体升高也可以作为化疗耐药性的预测因子[22]。临床实践中,超过一半的结直肠癌患者都有营养不良或有营养不良的风险,并存在营养与炎症状态之间的失衡[23],从而导致nCRT效果差。PNI被认为是反映治疗前营养与炎症的一个简单有效的指标,可以作为结直肠癌手术的预后预测因素[24],在开展nCRT之前,对存在营养失衡的患者进行治疗前的营养支持,改善患者的营养状态,可以在一定程度上增强患者对放化疗的耐受。COX-2抑制剂塞来昔布联合特瑞普利单抗相较于单用特瑞普利单抗可以显著提高pCR率[25],这或许可以为nCRT前的抗炎治疗提供新思路。
2004年报道的德国CAO/ARO/AIO-94研究[26]显示,nCRT比传统的辅助放化疗具有更高的局部控制率和更少的不良反应,因此确立了nCRT作为新的标准治疗策略。对于nCRT效果的预测,也有多项研究[16-17,27]相继进行过探索。无论是临床基线指标、影像学数据还是病理学数据对nCRT效果的预测能力都得到证实,但大多数研究侧重于预测患者的pCR率,而忽视了nCRT的客观缓解率。nCRT的根本目的是使肿块缩小,及早杀灭潜在的转移细胞,增加R0切除率和器官保存率。所以本研究的重点是nCRT后患者的肿瘤是否退缩和降期,能否获得手术机会及更高的R0切除率。在对比卡培他滨单药及XELOX两种化疗方案时,两组化疗方案并未显示出疗效差异,化疗方案的选择可能更多地取决于患者放化疗前的状态,双药方案更适合于治疗前状态好且能耐受更高化疗强度的患者。总体而言,nCRT后肿瘤均有不同程度的降期及可观的R0切除率。退缩不良组还有3例患者发生了远处转移,两组的肿瘤降期率和pCR率差异有统计学意义(P<0.05)。通过本研究的分类模型可以对nCRT后的肿瘤退缩程度进行有效的分类,对于预测nCRT后退缩不良的患者,可以根据临床实际情况调整放化疗方案或直接手术,避免或降低nCRT带来的不良反应,以达到最佳疗效。既往研究[28-29]的结果促进了全程新辅助治疗(total neoadjuvant therapy,TNT)研究设计,TNT是指在术前同步放化疗的基础上,将部分或全部的术后化疗前移到TME术前,期望通过增加化疗强度、延长手术等待时间,使得肿瘤退缩更为显著,这或许可以成为nCRT后退缩不良的局部进展期直肠癌患者的强化治疗方案,但目前尚未见相关报道。
本研究为nCRT后肿瘤退缩预测提供了一个简单有效的预测方案,但仍存在一定缺陷。首先,本研究是单中心回顾性研究,研究样本量较小,未进行前瞻性的验证;其次,研究观察的重点是肿瘤退缩程度,而肿瘤内部的生长和增殖情况未知,不能对远期预后进行评价。未来仍需要更大的样本量进行更深入的研究。
综上所述,治疗前肿瘤直径、nCRT后距离手术的时间、D-二聚体、PNI、CEA、EMVI是LARC患者nCRT后肿瘤退缩的独立风险因素,基于上述因素构建的肿瘤退缩预测模型对LARC患者的nCRT后肿瘤退缩具有较好的预测效能。
利益冲突声明:所有作者均声明不存在利益冲突。
作者贡献声明:
刘志昱:刘志昱完成了数据分析,进行了文献回顾和初稿撰写。
徐 栋:徐栋收集并完成了数据分析。
陈西昊:陈西昊参与了数据的收集。
李纪鹏:李纪鹏设计并指导了这项研究,对初稿进行了审核。
[参考文献]
[1] SUNG H, FERLAY J, SIEGEL R L, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249.
[2] National Health Commission of the People’s Republic of China, Chinese Society of Oncology. Chinese protocol of diagnosis and treatment of colorectal cancer (2023 edition)[J]. Chin J Surg, 2023, 61(8): 617-644.
[3] KENNEDY A, COHN M, COLDWELL D M, et al. Erratum to updated survival outcomes and analysis of long-term survivors from the MORE study on safety and efficacy of radioembolization in patients with unresectable colorectal cancer liver metastases[J]. J Gastrointest Oncol, 2018, 9(2): E13-E14.
[4] BENSON A B, VENOOK A P, AL-HAWARY M M, et al. Rectal cancer, version 2.2022, NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw, 2022, 20(10): 1139-1167.
[5] GÉRARD J P, CONROY T, BONNETAIN F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203[J]. J Clin Oncol, 2006, 24(28): 4620-4625.
[6] BOSSET J F, COLLETTE L, CALAIS G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer[J]. N Engl J Med, 2006, 355(11): 1114-1123.
[7] MAAS M, NELEMANS P J, VALENTINI V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data[J]. Lancet Oncol, 2010, 11(9): 835-844.
[8] AGARWAL A, CHANG G J, HU C Y, et al. Quantified pathologic response assessed as residual tumor burden is a predictor of recurrence-free survival in patients with rectal cancer who undergo rep after neoadjuvant chemoradiotherapy[J]. Cancer, 2013, 119(24): 4231-4241.
[9] MCCOY M J, HEMMINGS C, ANYAEGBU C C, et al. Tumourinfiltrating regulatory T cell density before neoadjuvant chemoradiotherapy for rectal cancer does not predict treatment response[J]. Oncotarget, 2017, 8(12): 19803-19813.
[10] YI Y X, SHEN L J, SHI W, et al. Gut microbiome components predict response to neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer: a prospective, longitudinal study[J]. Clin Cancer Res, 2021, 27(5): 1329-1340.
[11] EISENHAUER E A, THERASSE P, BOGAERTS J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1)[J]. Eur J Cancer, 2009, 45(2): 228-247.
[12] PARK I J, YOU Y N, AGARWAL A, et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer[J]. J Clin Oncol, 2012, 30(15): 1770-1776.
[13] ZENG W G, LIANG J W, WANG Z, et al. Clinical parameters predicting pathologic complete response following neoadjuvant chemoradiotherapy for rectal cancer[J]. Chin J Cancer, 2015, 34(10): 468-474.
[14] BUGG W G, ANDREOU A K, BISWAS D, et al. The prognostic significance of MRI-detected extramural venous invasion in rectal carcinoma[J]. Clin Radiol, 2014, 69(6): 619-623.
[15] MCCAWLEY N, CLANCY C, O’NEILL B D, et al. Mucinous rectal adenocarcinoma is associated with a poor response to neoadjuvant chemoradiotherapy: a systematic review and metaanalysis[J]. Dis Colon Rectum, 2016, 59(12): 1200-1208.
[16] SONG M, LI S, WANG H Z, et al. MRI radiomics independent of clinical baseline characteristics and neoadjuvant treatment modalities predicts response to neoadjuvant therapy in rectal cancer[J]. Br J Cancer, 2022, 127(2): 249-257.
[17] FENG L L, LIU Z Y, LI C F, et al. Development and validation of a radiopathomics model to predict pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: a multicentre observational study[J]. Lancet Digit Health, 2022, 4(1): e8-e17.
[18] BEDRIKOVETSKI S, TRAEGER L, VATHER R, et al. Clinical and biochemical predictors of tumor response after neoadjuvant therapy in rectal cancer[J]. Asia Pac J Clin Oncol, 2023, 19(3): 365-373.
[19] GAMBACORTA M A, MASCIOCCHI C, CHILOIRO G, et al. Timing to achieve the highest rate of pCR after preoperative radiochemotherapy in rectal cancer: a pooled analysis of 3085 patients from 7 randomized trials[J]. Radiother Oncol, 2021, 154: 154-160.
[20] HUANG W H, WANG S G, ZHANG H, et al. Prognostic significance of combined fibrinogen concentration and neutrophil-to-lymphocyte ratio in patients with resectable nonsmall cell lung cancer[J]. Cancer Biol Med, 2018, 15(1): 88-96.
[21] BEER J H, HAEBERLI A, VOGT A, et al. Coagulation markers predict survival in cancer patients[J]. Thromb Haemost, 2002, 88(5): 745-749.
[22] SHIBUTANI M, KASHIWAGI S, FUKUOKA T, et al. The significance of the D-dimer level as a prognostic marker for survival and treatment outcomes in patients with stage Ⅳ colorectal cancer[J]. In Vivo, 2023, 37(1): 440-444.
[23] MUSCARITOLI M, ARENDS J, BACHMANN P, et al. ESPEN practical guideline: clinical nutrition in cancer[J]. Clin Nutr, 2021, 40(5): 2898-2913.
[24] XIE H L, WEI L S, YUAN G H, et al. Prognostic value of prognostic nutritional index in patients with colorectal cancer undergoing surgical treatment[J]. Front Nutr, 2022, 9: 794489.
[25] HU H B, KANG L, ZHANG J W, et al. Neoadjuvant PD-1 blockade with toripalimab, with or without celecoxib, in mismatch repair-deficient or microsatellite instability-high, locally advanced, colorectal cancer (PICC): a single-centre, parallel-group, non-comparative, randomised, phase 2 trial[J]. Lancet Gastroenterol Hepatol, 2022, 7(1): 38-48.
[26] SAUER R, BECKER H, HOHENBERGER W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer[J]. N Engl J Med, 2004, 351(17): 1731-1740.
[27] MOMMA T, OKAYAMA H, KANKE Y, et al. Validation of gene expression-based predictive biomarkers for response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer[J]. Cancers (Basel), 2021, 13(18): 4642.
[28] GARCIA-AGUILAR J, CHOW O S, SMITH D D, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial[J]. Lancet Oncol, 2015, 16(8): 957-966.
[29] CERCEK A, ROXBURGH C S D, STROMBOM P, et al. Adoption of total neoadjuvant therapy for locally advanced rectal cancer[J]. JAMA Oncol, 2018, 4(6): e180071.